- Pidnarulex
- CX-5461
- G-quadruplex (stabilizer)
- Pidnarulex (CX-5461), a synthetically derived small molecule, is supplied as a lyophilized drug with intravenous (IV) administration.

Synthetic lethality (SL) is a concept in which cell death is induced by the combination of two non-lethal mutations, while mutation alone is not sufficient to affect cell survival. Recently, SL has been proposed as a novel anticancer strategy that is promising to be highly selective. If a cancer-specific mutation combined with a drug-induced mutation have SL interactions, it will selectively kill cancer cells but not normal cells.
For example, tumors with DNA repair defects, such as those arising from patients with BRCA mutations, were found to be more sensitive to PARP inhibition due to synthetic lethality. The BRCA genes encode large proteins with complex functions in DNA repair and cell cycle checkpoint control. Since BRCA mutated tumors cannot utilize homologous recombination (HR) to repair double-strand breaks, exposing these cells to a PARP inhibitor, which shuts down the base excision repair (BER) rescue pathway, will lead to the accumulation of DNA damage, genomic instability and cell death.

G-quadruplexes (G4) are four-stranded nucleic acid secondary structures that are over-represented in gene promoter regions and are viewed as emerging therapeutic targets in oncology. G4 sequences are highly prevalent in the human genome and are involved in DNA replication, gene expression and regulation, telomere/chromosome maintenance and genomic instability. When the G4 structure is stabilized by drug-like molecules, it may cause replication fork stalling, DNA breaks, and transcription–replication collisions, resulting in tumor cell death. Therefore, the development of novel stabilizers of G4 is an exciting anticancer approach in the potentially broad clinical applicability.

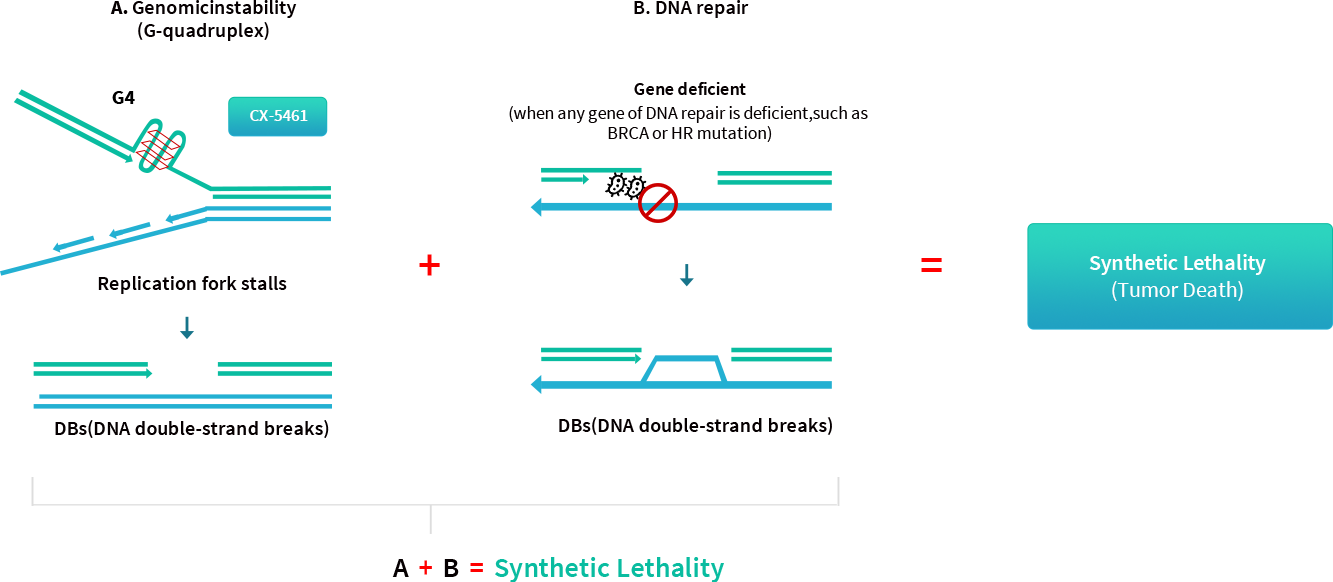
Synthetic lethality is defined as the combination of two non-lethal mutations that result in cell death. G-quadruplexes (G4) are higher-order DNA and RNA structures formed from guanine-rich sequences, and present in rapidly dividing cancer cells.
A. CX-5461 is a G4-stabilizing agent, which can stabilize the folded conformation and lead to replication fork stalling and unreplicated chromosomal areas. In turn, CX-5461 causes DNA breaks and triggers cellular DNA-repair mechanisms.
B. However, in cancer cells with defective homologous recombination (HR), such as BRCA1/2-deficient cells, double-strand breaks cannot be efficiently repaired due to the HR defect. This concept A+B is known as synthetic lethality.