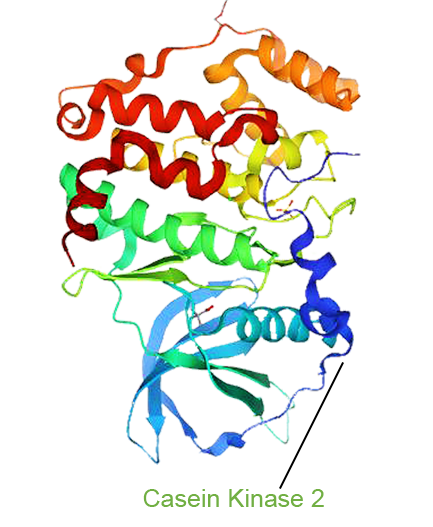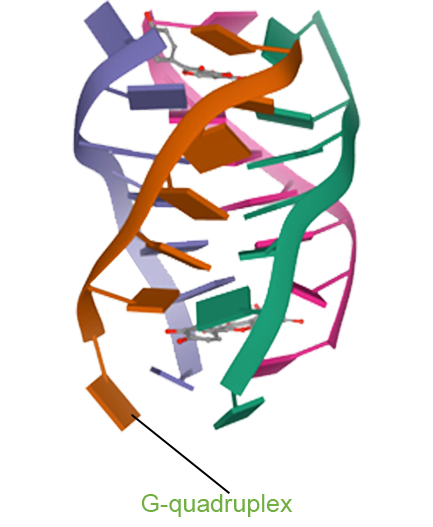- Bringing Hope to Life
- Senhwa is a precision therapy company focusing on developing First-in-Class therapeutics for patients with unmet medical needs. These include multiple programs for gene-mutated cancers, rare diseases, and novel coronaviruses.
- OUR PIPELINE
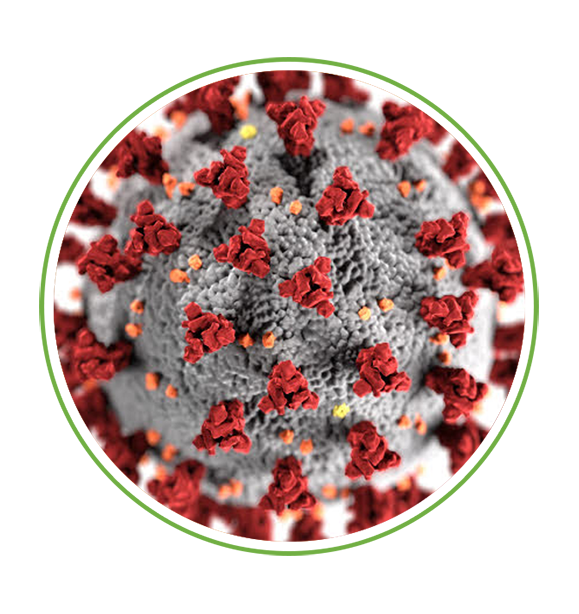
- Coronavirus Curative
- The first patient treated with Senhwa’s Silmitasertib (CX-4945) for severe COVID-19 demonstrated remarkable recovery and was discharged in 5 days after treatment.
- OUR CLINICAL TRIALS
- One Drug with Multi-target Therapy
- Senhwa’s Pidnarulex (CX-5461) has proven to be the next generation DDR (DNA Damage Response) drug, with the mechanism of synthetic lethality across multiple tumor types in patients with loss of function of tumor suppressor genes such as HRD (Homologous Recombination Deficiency) and beyond, and has great potential for patients who have developed resistance to PARP inhibitors and platinum or other chemotherapies.
- OUR PLATFORM

- Pioneering the Paradigm
- Senhwa strives to design and develop innovative therapies rooted in our leading expertise in protein kinases inhibition, and improve quality of life for cancer patients. .
- OUR COMPANY