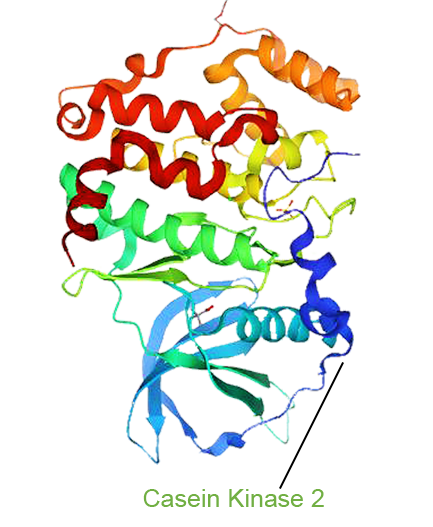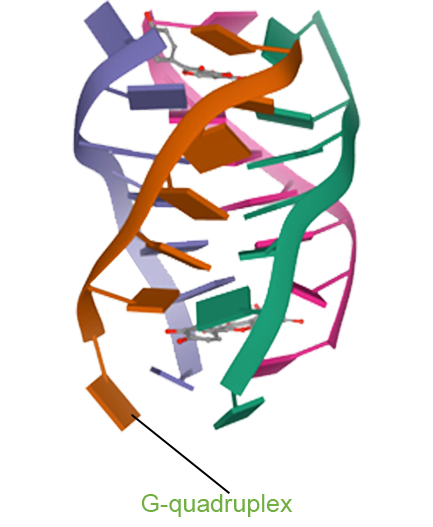- Bringing Hope to Life
- Senhwa is dedicated to developing first-in-class drugs to address unmet medical needs, bringing new hope to patients. Currently, the company is advancing multiple innovative programs, including a five-year cancer research project sponsored by the U.S. National Cancer Institute (NCI), focusing on monotherapy, combination cancer immunotherapy (IO), and antibody-drug conjugates (ADC). Additionally, Senhwa focuses on cancers caused by specific gene deficiencies, rare diseases, and abnormal inflammatory responses linked to viral infections.
- OUR PIPELINE
- A Potential Game Changer Toward a Functional Cure for HIV
- Silmitasertib (CX 4945), an oral CK2 inhibitor from Senhwa Biosciences, disrupts phosphorylation of key HIV proteins (Tat, Rev, Nef), which are involved in viral replication, latency and immune evasion. It also targets CK2’s role in immune dysregulation linked to chronic inflammation and T cell exhaustion. By targeting both active virus and the dysfunctional immune environment, Silmitasertib shows promise as a candidate for a functional HIV cure.
- Turn Cold Tumors into Hot Ones
- Pidnarulex (CX-5461) has demonstrated the potential to convert “cold tumors” into “hot tumors” by altering the tumor microenvironment, thereby enhancing the therapeutic response to immune checkpoint inhibitors (ICIs). Preclinical studies confirm that Pidnarulex induces immunogenic cell death, activating immune cells and improving the tumor microenvironment. When combined with immunotherapy, Pidnarulex holds promise for treating a variety of cancers resistant to ICI monotherapy.
- PUBLICATIONS

- Development of Innovative Orphan Drugs
- Silmitasertib (CX-4945) has obtained four orphan drug designations, including for cholangiocarcinoma, biliary tract cancer, medulloblastoma, and neuroblastoma. It has also received rare pediatric disease designation for the treatment of medulloblastoma and neuroblastoma. The future market launch will benefit from advantages such as tax credits and a seven-year exclusive market period in the United States.
- OUR CLINICAL TRIALS